back to Training for the Medical Device Industry
back to Training for the Medical Device Industry
Risk Management for Medical Device Manufacturers
Many medical device manufacturers may find it difficult to perform Risk Management when software is embedded within their medical device OR when software IS the medical device. This course can help your organization learn effective techniques for performing Risk Management on devices with embedded software. In addition, this course will illustrate ways to integrate Risk Management activities throughout your Quality Management System (QMS).You will learn to apply powerful tools such as:
- Fault Tree Analysis (FTA)
- Failure Modes Effect and Criticality Analysis (FMECA)
- Software Reliability Growth Modeling
This course can help identify areas of potential regulatory exposure. By applying the principles learned in this course, you'll be able to improve the effectiveness of your development activities, measure and improve product reliability, minimize the likelihood of potentially costly product recalls, and significantly reduce rework and product maintenance costs.
Topics covered include:
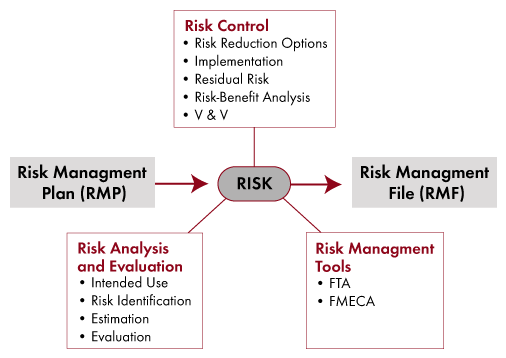
Risk Management Overview
- Overview of Regulatory Requirements and Standards including: ISO 14971, IEC 62304, and ISO/TR 80002-1
Risk Concepts and Terminology
- Risk Assessment
- Identifying Known or Foreseeable Hazards
- Estimating Risk
Risk Control Techniques
- Inherently Safe Design
- Hazard Detection
- Information for Users
Risk Management Tools
- Fault Tree Analysis: The top-down approach
- Failure Mode Effects Criticality Analysis (FMECA)
- System Hazard Analysis using Fault Trees and FMECAs
- Verification and Validation of Mitigations
Exercise: Fault Tree Analysis Integrating Risk Management into the QMS
- Risk Management File
- Measuring Software Reliability Growth